Assignment Prompt
This will cover the electrophilic aromatic substitution reaction: Friedel–Crafts alkylation of p-xylene. Discuss the mechanism of the reaction in your introduction, including an explanation of ortho/para- and meta-directing groups. Use your GC/MS data to determine which product(s) were obtained for the alkylation experiment. Explain why each product is formed in your results and discussion.
Here are some hints for analyzing your GC/MS data:
You added excess p-xylene. Look up its molar mass and identify that peak in your GC/MS. It should have a high-abundance peak for the fragment formed when one methyl group is lost. There are two expected isomers of the alkylation product: one where rearrangement occurs and one where it does not. They should have the same molar mass (approximately 148 g/mol), but they should have different fragments.
2-isopropyl-1,4-dimethylbenzene should have a fragment with a mass of 133 g/mol.
1,4-dimethyl-2-propylbenzene should instead have a fragment with a mass of 119 g/mol.
Alkylation can happen more than once on the same aromatic ring, even when the ring is in excess. You should see peaks in the mass spectrum that indicate this occurring. Calculate the molar mass of dimethyldipropylbenzene and identify those peaks. Also calculate the molar mass of dimethyltripropylbenzene; this mass is found in the peak(s) around 11 minutes. These peaks are quite small and can be ignored for identification purposes, but they are evidence that the ring continues to react even after two new alkyl groups are added. You should explain why this happens in the results and discussion.
Carbocation Rearrangements and Product Distributions in Friedel-Crafts Alkylation of p-Xylene
Abstract:
This experiment examined the Friedel-Crafts alkylation of p-xylene with 1-chloropropane using AlCl3 catalyst to study electrophilic aromatic substitution and carbocation rearrangements. The reaction yielded two isomeric products identified by GC/MS: 1,4-dimethyl-2-propylbenzene (119.1 amu) and 2-isopropyl-1,4-dimethylbenzene (133.1 amu). Minor peaks at 161.2 and 175.2 amu revealed disubstituted byproducts, which suggests continued ring reactivity due to methyl group activation. The products eluted in boiling point order, with the lower-boiling isopropyl isomer with a boiling point of 196.2°C preceding the linear propyl analog whose boiling point is 204°C. The major limitation was that quantitative ratios could not be determined without peak areas. Nonetheless, it was determined that there was successful monoalkylation with rearrangement, electronic effects governed regioselectivity, and over-alkylation was observed despite steric constraints. These results align with the mechanistic principles of Friedel-Crafts reactions, including carbocation stability and substituent-directed reactivity in aromatic systems.
Introduction
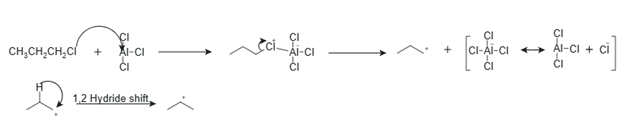
Figure 1 Reaction mechanism illustrating (a) AlCl3 activation of 1-chloropropane, (b) formation of the primary propyl cation and its hydride shift to the secondary isopropyl cation
Due to the rearrangement of the electrophile, electrophilic aromatic substitution on a ring that contains a substituent results in isomeric products. For this experiment, two possible monosubstituted products are obtained: 1,4-dimethyl-2-propylbenzene and 2-isopropyl-1,4-dimethylbenzene, which result from rearrangement as shown in Figures 2 and 3 below. Methyl groups donate electron density through hyperconjugation and inductive effects, making the ring more nucleophilic and accelerating the reaction. The existing substituents influence where electrophilic attack occurs, leading to regioselectivity. The first group of substituents is the ortho-/para-directing group. These are electron-donating groups such as CH3, OH, and NH2, which activate the ring and push electron density toward the ortho (1,2) and para (1,4) positions.2 As such, the two methyl groups on p-xylene are strong activators, favoring substitution at these locations. The second group is the meta-directing groups, which are electron-withdrawing groups such as NO2, CN, and CF3. These groups pull electron density away from the ring, favoring substitution at the meta (1,3) position.2 Since p-xylene contains electron-donating methyl groups, meta-substitution is unlikely for this experiment.
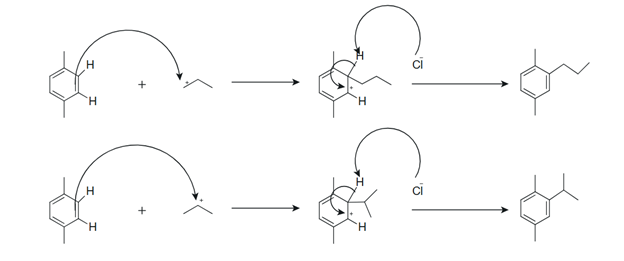
Figure 2 Reaction mechanism illustrating electrophilic attack on p-xylene by the primary propyl cation and stable isopropyl cation
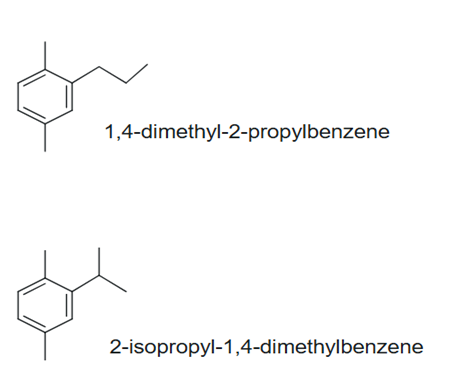
Figure 3 The Reaction Products: 1,4-dimethyl-2-propylbenzene and 2-isopropyl-1,4-diimethylbenzene
Experimental
A 25-mL round-bottom flask with a stir bar was added 3.7 mL (3.19 g, 0.030 mol) of p-xylene. A Claisen adapter was attached to the flask, with a rubber septum fitted to the straight arm and a drying tube containing CaCl2 connected to the curved arm. Afterward, 0.20g (0.0015 mol) of anhydrous AlCl3 was quickly weighed and added to the flask to minimize exposure to atmospheric moisture. The mixture was stirred, and 0.14 mL (0.118 g, 0.0015 mol) of 1-chloropropane was added using a syringe through the septum. This was done for 2 hours, during which the solution transitioned from the initial yellow to orange.
For workup, 4 mL of water was added to quench residual catalyst before transferring the mixture to a receiving flask. The aqueous layer was then removed via pipette, and the organic layer was washed sequentially with approximately 3 mL of 5% NaHCO3 and about 3 mL of water. The organic phase was dried over Na2SO4, decanted into a clean vial, and stored for GC/MS analysis. The total mass of the crude product was 10.91 g, while the theoretical yield was determined to be 0.225 g (0.0015 mol) of dimethylpropylbenzene isomers.
GC/MS analysis was performed to identify the products, where the results showed early-eluting peaks at 91.1 amu, followed by 119.1 amu, then 133.1 amu, with later-eluting minor peaks at 161.2 amu and 175.2 amu. The tallest peak (91.1 amu) eluted first, while the rearranged product (133.1 amu) appeared after the non-rearranged isomer (119.1 amu). The highest-mass fragments (161.2 amu, 175.2 amu) eluted last. No significant peaks were observed beyond this range.
Results and Discussion
The GC/MS analysis of the crude product mixture revealed several distinct components. To start with, the prominent early-eluting peak at 91.1 amu, as shown in Appendix A below, was identified as unreacted p-xylene since it corresponds to the tropylium ion commonly observed in alkylbenzenes. In the mid-range of the chromatogram, two peaks were observed, each displaying a molecular ion at approximately m/z 148.1, as shown in appendices B and C, consistent with monosubstituted products of the Friedel–Crafts alkylation. This confirmed that they are constitutional isomers of the same molecular formula. One of these spectra exhibited a base fragment at m/z 119.1, as shown in appendix C while the other showed a dominant peak at m/z 133.1 as shown in appendix B. The observed mass fragments confirm the formation of both possible monosubstituted products. The peak at 119.1 amu represents the non-rearranged product (1,4-dimethyl-2-propylbenzene), formed through direct attack of the primary propyl cation. In contrast, the more intense peak at 133.1 amu corresponds to the rearranged product (2-isopropyl-1,4-dimethylbenzene), resulting from the more stable secondary isopropyl cation.
A minor later-eluting peak showed a molecular ion at m/z 175.2, as shown in appendix D below, indicating the presence of a disubstituted product, most likely dimethyldipropylbenzene, with a secondary fragment at m/z 161.2. However, no significant peaks were observed near m/z 232, suggesting negligible formation of tripropylated products. These result from further alkylation even after two new alkyl groups are added. This happens because alkyl groups are electron-donating substituents that activate the aromatic ring by increasing its electron density through both inductive and hyperconjugative effects. This enhanced nucleophilicity makes the ring more reactive toward subsequent electrophilic attacks.
References
(1) Mąkosza, M. Electrophilic and Nucleophilic Aromatic Substitutions are Mechanistically Similar with Opposite Polarity. Chemistry - a European Journal 2020, 26 (67), 15346–15353. https://doi.org/10.1002/chem.202003770.
(2) Libretexts. Substitution reactions of benzene derivatives. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Arenes/Reactivity_of_Arenes/Substitution_Reactions_of_Benzene_Derivatives.
Paper Successfully Completed
This sample paper has been professionally written and meets all academic standards.
