Introduction to Lab Reports
We have all been there before! You’ve just spent three hours in the lab, and now your fingers are stained, you’ve smelled enough chemicals to last a lifetime, and your notebook is a chaotic masterpiece of numbers and scribbles. But all this means nothing if you do not get a good grade from the lab report write-up. The challenge is that you have no idea how to deliver a good lab report that gets the highest grade. Maybe your professor never taught lab report format, or you just forgot, but it does not matter.
In this article, I intend to be your lab report coach: I will introduce the lab report format, discuss the sections, including the lab report title page or cover page, abstract, introduction, materials and methods, results, discussion, and conclusion. We will use examples from a sample lab report in chemistry to drive home the points.
I will also attach a lab report template you can use to ensure you get all marks related to formatting. Other than that, I will answer some of the questions you might have, including "how to write an abstract for a lab report", "how to write a conclusion for a lab report", "how to format a lab report", and "how long should a lab report be". So let us get to it!
The Standard Lab Report Format
Unlike other forms of writing, lab reports are structured to mirror the scientific method. This is the format for a standard lab report: (1) Abstract, (2) Introduction, (3) Methods, (4) Results, (5) Discussion. This is called the IMRaD format.
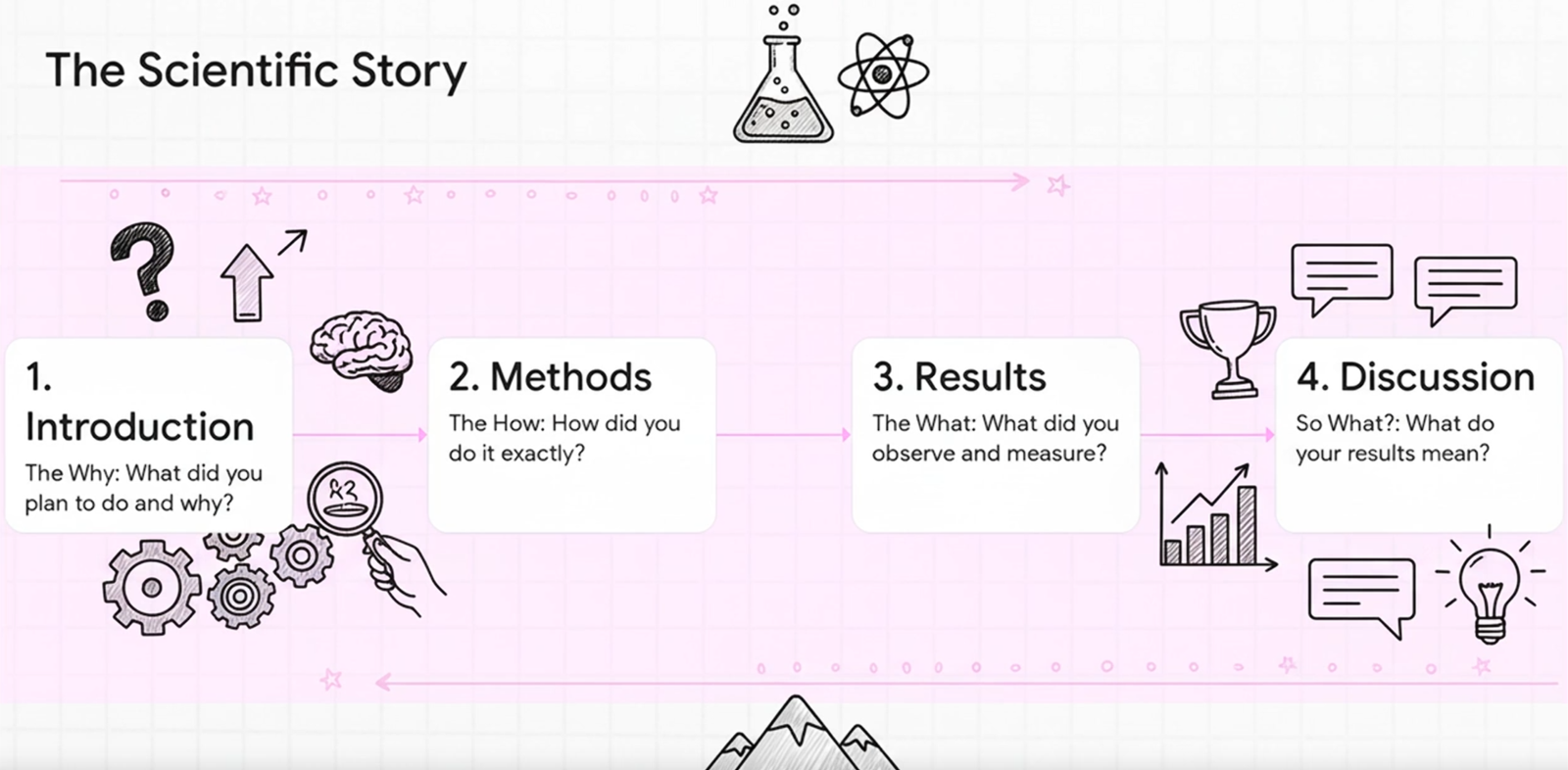
IMRaD stands for:
-
- Introduction: What did you plan to do and why?
- Methods: How did you do it exactly?
- Results: What did you observe and measure?
- and Discussion: What do your results mean?
You might have noted that the IMRaD structure does not include an abstract or a conclusion. This is because the abstract is not really part of the paper, but should be included if your professor/TA requires it or if the word count does not restrict you. Please note that the abstract is not optional for higher levels of education. The conclusion, on the other hand, comes as the last paragraph of your discussion. We will talk about that in depth later!
How to Format a Lab Report
Now, let's talk about the universal rules for how to format a lab report. These are the non-negotiable style points that make your work look professional:
- Font & Spacing. Use a readable, serif font like Times New Roman (12pt) or a clean sans-serif like Arial (11pt). Unless your professor or TA says otherwise, your entire document should be double-spaced to leave room for comments. Single-spaced reports are an immediate eyesore.
- Margins. Stick to 1-inch margins on all sides. This provides a clean frame for your text.
- Page Numbers. Place page numbers in the top right corner, starting on the title page as page 1.
- Headings. Use bolded headings (like Introduction, Methods) to clearly separate each section. I find that the general rules for headings in APA 7 apply well to lab reports.
- Voice and Tense. This is crucial. Write in the third person and the past tense. You are reporting on work that was already completed. Avoid: "I will heat the mixture to 50°C." or "We see that the color changes." Instead, use: "The mixture was heated to 50°C." or "The color changed from clear to blue."
- Units & Notation. Every numerical value gets a unit. 23.5 is meaningless; 23.5 mL is precise. Use proper scientific notation (e.g., 6.022 x 10²³) and significant figures consistently.
- References. Your work must be supported by peer-reviewed scholarly work. I always like it better when my students use ACS style for citations. I know others prefer other styles such as APA or MLA. You can check out my guide on these styles.
Lab Report Format: The Key Sections in Detail
You can use a lab report on Carbocation Rearrangements and Product Distributions in Friedel-Crafts Alkylation of p-Xylene as the example for you to follow along.
The Lab Report Title Page / Lab Report Cover Page
This is the first page of your paper. Think of your lab report cover page as the suit and tie for your report. It's the first thing seen, and a messy one creates a poor first impression. Its sole job is to provide flawless identification. So, what should you include and why?
- The Title: This is your one chance to summarize the entire experiment. Be specific and descriptive. Here is an example of a good title: “Carbocation Rearrangements and Product Distributions in Friedel-Crafts Alkylation of p-Xylene.” Avoid a generic title like “Carbocation Rearrangements” or worse, “Synthesis Lab.
- Your Name & Partners' Names: List all contributors alphabetically by last name or as per your instructor's preference. Include student IDs if required.
- Instructor's Name: Use their proper title (e.g., Dr. Alanna Rodriguez, Professor James Chen).
- Course Information: The full course name and code (e.g., CHEM 110L: General Chemistry I Laboratory).
- Dates: Include both the Date the Experiment was Performed (e.g., September 26, 2023) and the Date of Submission (e.g., October 3, 2023). This is important for context.
- Lab Section: Your specific lab section or group number (e.g., Section L04, Group A).
See the example below:
Abstract: How to Write an Abstract for a Lab Report
The abstract is a single, dense paragraph (typically 150-250 words) that sits alone on its own page after the title page. It is a miniature version of your entire report. Imagine a busy professor reading only this, they should get the complete story. It, therefore, follows that you can only write it after you have completed the report, not before! Follow these steps to write a strong abstract:
- State the broad purpose and the specific goal in 1 sentence. Example: “The purpose of this experiment was to apply the principles of acid-base chemistry to quantitatively determine the molar concentration of acetic acid present in a sample of commercial white vinegar.”
- Briefly describe the core experimental technique, without listing details in 1-2 sentences. Example: “This was accomplished through a titration, where the vinegar sample was neutralized with a precisely known concentration of sodium hydroxide (NaOH) solution, using phenolphthalein to identify the reaction endpoint.”
- Present the most important numerical findings with units and a sense of precision/uncertainty in 1-2 sentences. Example: “The average concentration of acetic acid was found to be 0.847 M with a standard deviation of ±0.012 M across three trials. This corresponds to a mass percentage of 5.07%.”
- State what the result means in relation to the objective or a broader context in 1 sentence. Example: “This value is in strong agreement with the typical acetic acid content in household vinegar (5.0%), thereby validating the titration method for accurate quantitative analysis and confirming the product's labeled specification.”
Remember to always write the abstract last!
Introduction
In this section, you need to answer three questions: What is the broader scientific context? What specific theory are you using? What exactly are you trying to find out?
Always start with the general chemistry principle at play: “Electrophilic aromatic substitution (EAS) reactions represent one of the most important reaction classes in organic chemistry, allowing for the functionalization of aromatic rings. They involve the replacement of a hydrogen atom on an aromatic ring using an electrophile while preserving the aromatic π‐system. These reactions are facilitated by the π electron clouds of aromatic rings, which make them suitable recipients of electrophilic agents.1” Note the citation.
In the next paragraph, narrow your focus to the specific method used in the lab. Define key terms and introduce the governing chemical equation. This is where you demonstrate your theoretical understanding. Include this equation, centered on its own line.
In the last paragraph, clearly and concisely state what this specific experiment set out to do. If applicable, state a testable prediction. Example: “The aim of this experiment was to determine the molarity and mass percentage of acetic acid in a sample of commercially available vinegar. Based on the product label stating '5% acidity,' it was hypothesized that the titration would yield an acetic acid concentration of approximately 0.83 M.”
Methods (or Experimental)
This section is a description of what you specifically did. Write it so that a competent peer in your class could pick up your report and repeat the experiment exactly, obtaining the same results. Write in full sentences, in past tense, detailing your specific actions.
Example: "A 50 mL buret was rinsed with three small portions of the standardized NaOH solution and then carefully filled. An initial volume reading was recorded to the nearest 0.01 mL. Using a volumetric pipette, 25.00 mL of the vinegar sample was transferred into a clean 250 mL Erlenmeyer flask. Two drops of phenolphthalein indicator were added to the flask, producing a colorless solution. The vinegar was then titrated by slowly adding NaOH from the buret while continuously swirling the flask. The titration was stopped when the addition of a single drop produced a faint pink color that persisted for at least 30 seconds of swirling. The final buret reading was recorded. This procedure was repeated for two additional trials."
Briefly note any significant safety precautions e.g., "Safety goggles were worn at all times" and disposal procedures e.g., "All neutralized waste was disposed of in the designated aqueous waste container".
Results
The Results section is for data only—no opinions, no explanations, no theories. Your job is to present the cleaned and processed data clearly using text, tables, and figures.
- Processed Data Tables: The data must be processed, never present raw notebook scribbles. Create clean, labeled tables with calculated values. See the images below.
- Sample Calculations: Show the math for one representative set of data. You can include this in the text or in an appendix, I prefer it in text.
- Figures/Graphs: Where applicable, like a spectroscopy lab, you would include a calibration curve here with a proper title, labeled axes, and a trendline equation.
Discussion
This is where you prove you understand the science, not just the procedure. It's the most important section for earning a high grade. Connect your results back to the theory from the Introduction and analyze what happened.
- Paragraph 1: Restate & Interpret Key Result. Begin by directly addressing your hypothesis with your data.
- Paragraph 2: Analyze Error & Uncertainty. Discuss the percent error (vs. the expected value) and the precision of your trials (standard deviation). Propose specific, plausible sources of error. This shows critical thinking.
- Paragraph 3: Connect to Theory & Explain Observations. Go deeper. Why did the color change occur? What does the sharpness of the endpoint tell you?
- Paragraph 4: Discuss Limitations and Broader Implications. What could be improved? Why does this matter outside the lab?
Conclusion
The conclusion is not a rehash of the discussion. It is a brief, powerful, and final statement of what was learned. Purpose: To concisely answer the question, "Taking everything into account, what is the ultimate takeaway?" Write a 3-4 Sentence Conclusion:
- Concisely restate the overall aim.
- State the final result and whether the hypothesis was supported.
- Briefly mention the quality of the data (accuracy/precision).
- Suggest ONE logical, practical next step or improvement.
Your Final Pre-Submission Checklist
Print this and check each box before you hand in any report:
- Lab Report Cover Page/Title Page is perfectly formatted with all required information.
- Abstract is a single paragraph on its own page, written last, and follows the Objective-Methods-Results-Conclusion formula.
- Introduction flows from general to specific, includes key theory/equations, and clearly states the aim/hypothesis.
- Methods are written in past tense, narrative form, and are detailed enough for replication.
- Results present data in clear, labeled tables/figures; sample calculations are shown; the text describes trends without interpreting them.
- Discussion interprets results, calculates and explains error, connects back to theory, and explores implications.
- Conclusion provides a final, concise takeaway that answers the "so what?" question.
- References are properly cited in a consistent style (e.g., APA, ACS).
- Entire Document follows formatting rules: double-spaced, 1-inch margins, 12pt font, page numbers, third-person/past tense.
Final Thoughts
Writing a stellar lab report is a skill, and like any skill, it gets easier with practice and a good guide. You now have that guide. You understand the logic of the lab report format, have a detailed lab report template to follow, and know exactly how to write an abstract and conclusion that pack a punch.



Comments (0)
Add Your Comment
No comments yet. Be the first to comment!